MENINGOCOCCAL STUDY
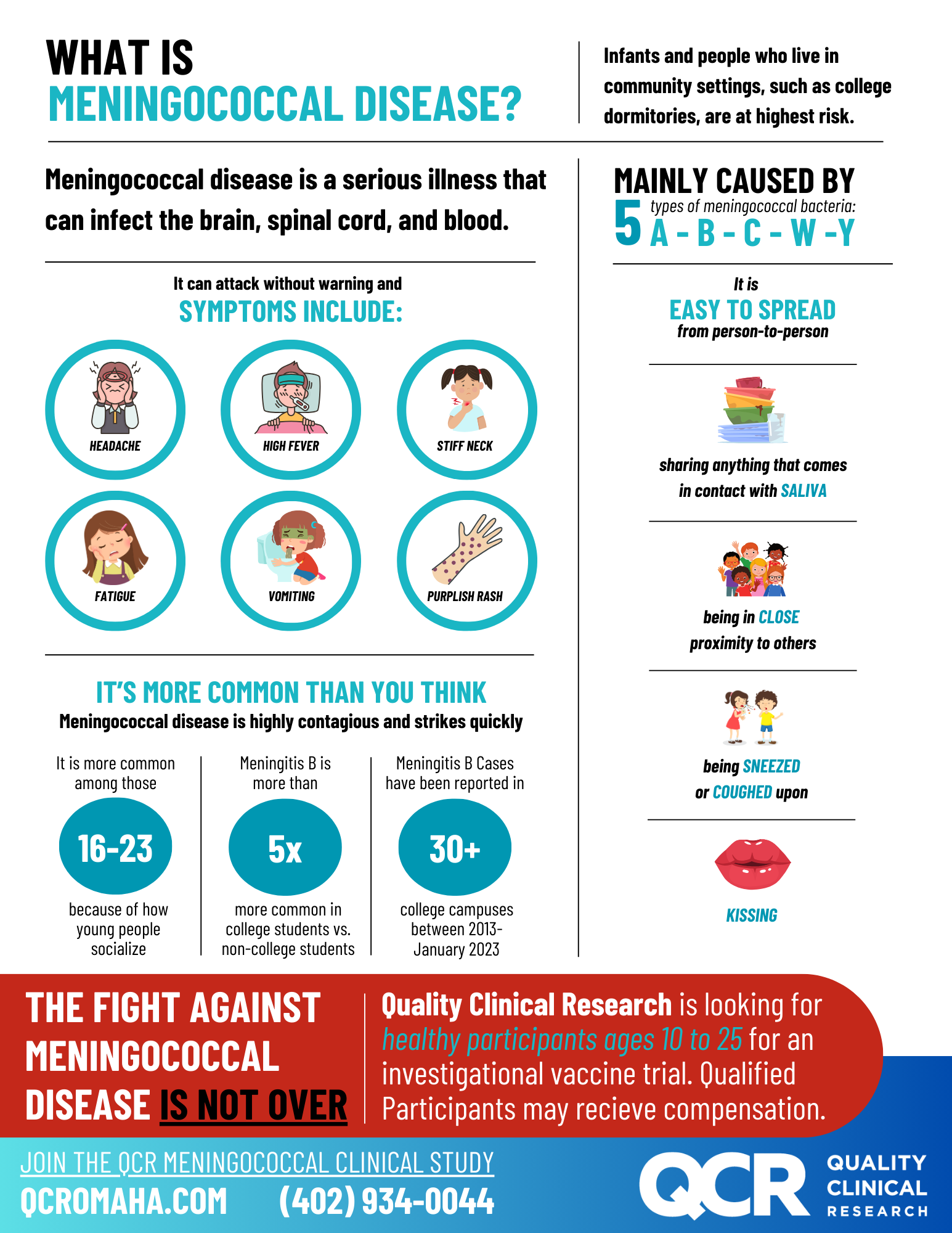
The purpose of this clinical research study is to assess the safety and immunogenicity of the investigational MenPenta vaccine in adults and adolescents.
The MenPenta vaccine is developed as a combination vaccine using meningococcal ACYW-TT conjugate antigens of licensed MenQuadfi® vaccine and the investigational multicomponent meningococcal B antigens (Sanofi MenB vaccine) that is intended to provide protection against the five most epidemiologically relevant neisseria meningitidis serogroups worldwide.
To qualify for the study, you must be:
- Aged 18 to 25 years or 10 to 17 years on the day of inclusion
- Participants who are overtly healthy as determined by medical evaluation including medical history, physical examination, and judgment of the investigator.
- Other criteria may apply*
*There are multiple studies available to participate in and each may require different criteria to be approved as a participant.
To learn more about this trial and to find out if you qualify, please call 402.934.0044 or complete the form on our Contact page.